Research on cystic fibrosis (CF) at Boston Children’s touches every aspect of the disease and its care. As one of the largest cystic fibrosis centers in the United States, we are a major hub for clinical drug trials, enrolling people of all ages. We also study many non-drug interventions for CF and conduct extensive research aimed at improving quality of life, through the Success With Therapies Research Consortium, as well as maximizing self-care at home. We have also launched gene therapy trials that could potentially provide benefit for those who cannot take CFTR modulator drugs.
Through dedicated research staff, specialized equipment, and our extensive research infrastructure, we are able to participate in virtually any study, and will work with you to identify studies for which you may be eligible.
We also have an active laboratory research program to investigate how CF works at the cell and tissue level and discover new and better treatments. We invite anyone to participate in our Pulmonary Bank Initiative to help advance these efforts. A member of our research team may approach you about enrolling during one of your visits.
Cystic fibrosis research: Clinical studies
CF drug trials
Through the CF Foundation’s Therapeutics Development Network, we participate in numerous clinical drug trials, enrolling adults, children, infants, and even newborns. Treatments studied include:
- new CFTR modulators (drugs that boost function of the CFTR protein that is altered in CF)
- inhaled antibiotics and bacteriophages (viruses that kill bacteria) to treat lung infections
- chloride channel openers
- anti-inflammatory drugs
- treatments for complications such as pancreatitis
Non-drug interventions for cystic fibrosis
Interventions we’re studying include:
- nutritional interventions to improve growth, such as web-based applications
- evaluations of video-based coaching and smartphone apps to help make treatment regimens easier to follow
Studies aimed at improving quality of life in cystic fibrosis
These studies include:
- the recently completed SIMPLIFY trial, assessing whether people taking CFTR modulators can safely stop some of their other CF treatments
- studies of stress and coping in people with CF
Gene therapy trials for cystic fibrosis
We are in the early stages of testing a gene therapy that would deliver a healthy CFTR gene specifically to cells in the lungs, for people with CF who cannot tolerate CFTR modulator drugs. More information.
Observational studies
Observational studies help us better understand how cystic fibrosis affects people day-to-day and over time. Examples include:
- the development of lung infections and other complications of CF
- how CFTR modulator treatment affects bone density
- how CFTR modulator treatment affects the sinuses and smell function
- the effects of diet and exercise on lung health in CF
- the safety and effectiveness of hormonal contraceptives for women with CF
- pregnancy outcomes in women with CF and their babies
Studies of innovative devices
Devices we’re evaluating include:
- a “bionic pancreas” for automated glucose management in CF-related diabetes
- home spirometers for measuring lung function
- vests that deliver chest physiotherapy at home
See a list of all clinical studies through the CF Foundation’s clinical trials finder. You can use the filters to narrow your search — for example, entering “Boston” and checking off the status “enrolling.” You can also feel free to check with our staff at any time.
Cystic fibrosis research: Laboratory studies
The Boston Children’s Pulmonary Bank Initiative has gathered DNA samples from more than 600 patients with lung disease who are interested in participating in research, including a growing number of people with cystic fibrosis. These samples, typically obtained from the blood or a cheek swab, can be used to better understand CF and genetic factors that may explain the variability in lung function from person to person.
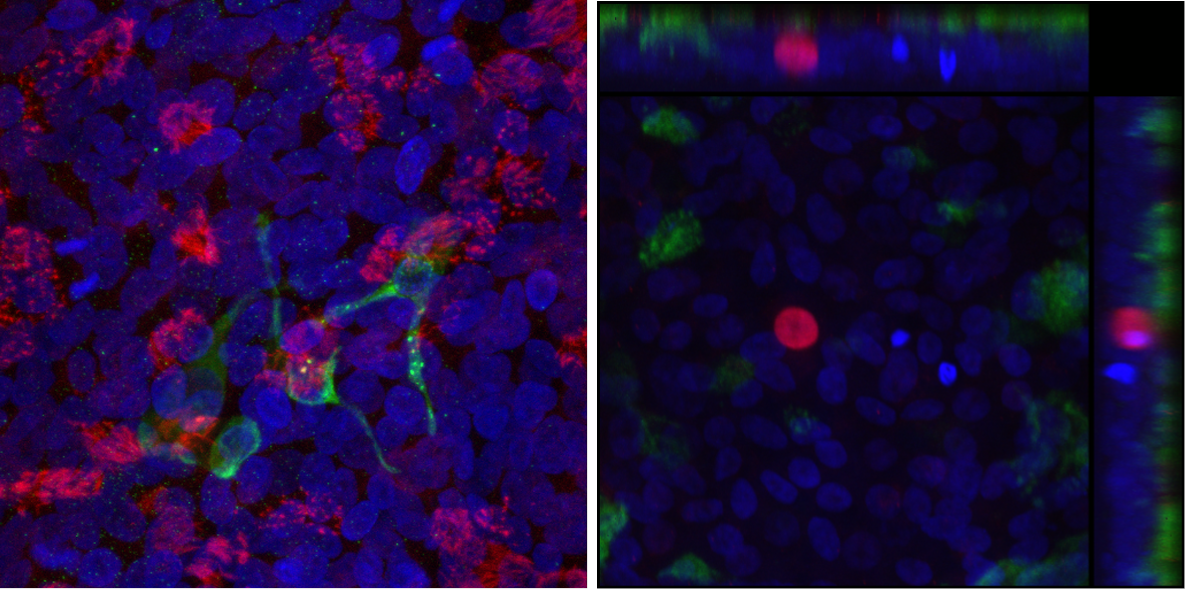
At left, a model airway derived from patients’ cells that can be used to test drugs. It contains ciliated respiratory cells, some of which are infected with the SARS-CoV-2 coronavirus. At right, three pulmonary ionocytes, a rare airway cell type, derived from a CF patient’s blood cells via stem cell technology. (Courtesy Dr. Ruby Wang/Boston Children’s Hospital)
These studies include:
‘Living’ airway models of cystic fibrosis
Using blood cells from people with CF, our researchers have constructed model airways that contain all the key cell types lining the trachea and bronchi. These “organoids” can be used to study how infections like COVID-19, flu, and Pseudomonas aeruginosa affect the airways of people with CF, without the need for invasive tissue sampling, and how the effects change when the CFTR gene is edited. The model can also be used to test different treatments.
Studies of ionocytes
Ionocytes make up less than 1 percent of the cells lining the respiratory tract, yet they produce more than 90 percent of the CFTR protein. For the first time, our researchers have been able to generate ionocytes from the blood cells of patients with CF, first converting them into lung stem cells and then directing the stem cells to form ionocytes. Our team is studying these cells to understand what role they play in the respiratory tract, and whether targeting them would be effective way to treat CF.
Understanding ‘extreme phenotypes’ of cystic fibrosis
Some people with CF have preserved lung function into their 60s and 70s, while others need lung transplants at a young age — yet all have the CFTR mutation. A research team is using genetics and stem cell models to understand if there are genes that modify the effects of CFTR in these CF “outliers.” They hope to get insights that will lead to new treatments.
Cell therapies for cystic fibrosis
Another team at Boston Children’s is exploring cell therapy as a way to rehabilitate damaged lung tissue without the need for lung transplant, by introducing healthy, genetically edited lung stem cells created from patients’ own cells. So far, this general approach shows signs of working in a mouse model.
