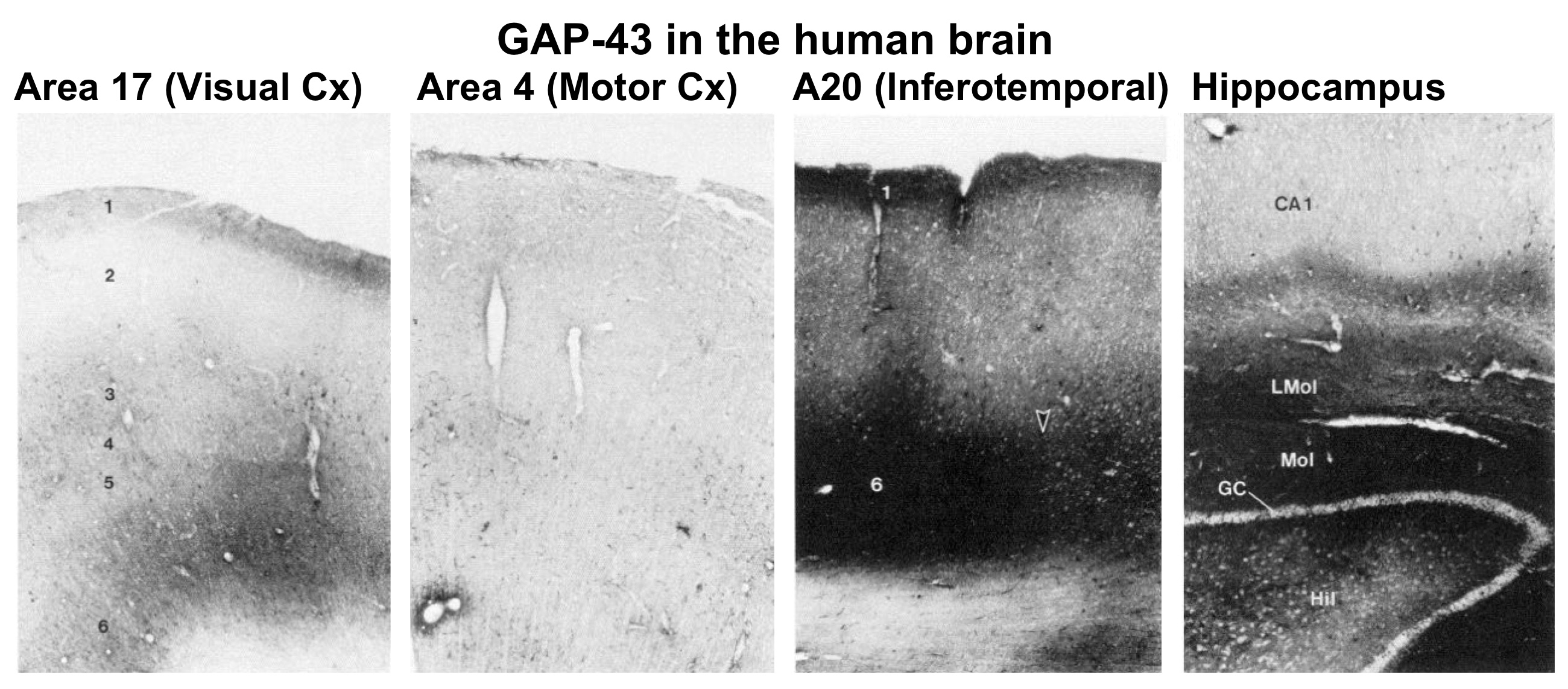
Unlike mammals, fish and amphibia can readily regenerate their optic nerves, restore a topographic map of visual space in the brain, and recover vision. Because membrane-associated proteins are transported down axons far more rapidly than cytoskeletal proteins and other components, we utilized double-isotope labeling and one- and two-dimensional gel electrophoresis to identify rapidly transported proteins that might be involved in axon elongation and in determining the properties of growth cones and early synapses. This work led to the (co)-discovery of the protein that came to be called GAP-43 (Benowitz et al., 1981; Benowitz and Lewis, 1983; Benowitz et al., 1983). We showed that this protein was enriched in the membranous fraction of nerve terminals during axon growth and that probes to GAP-43 mRNA and protein provided novel in-sights into the developmental time-course of the peripheral and central nervous system (Moya et al., 1989; Erzurumlu et al., 1990; Dani et al., 1991; Fitzgerald et al., 1991; Reynolds et al., 1991). We showed that GAP-43 was phosphorylated by a particular isoform of protein kinase C (Schaechter and Benowitz, 1993), and that it was identical to a protein identified in other contexts as an activity-regulated phosphoprotein in certain synapses (Perrone-Bizzozero et al., 1986). We also showed that, whereas levels of GAP-43 decline in most brain areas in the early postnatal period, in the rat and human brains, it remained highly expressed in the hippocampus and associative areas of cortex, where it may be involved in ongoing structural and/or functional plasticity (Neve et al., 1987; Benowitz et al., 1988; Neve et al., 1988; Benowitz et al., 1989).
References
- Benowitz LI, Shashoua VE, Yoon MG (1981) J Neurosci 1:300-307.
- Benowitz LI, Lewis ER (1983) J Neurosci B:2153-2163.
- Benowitz LI, Yoon MG, Lewis ER (1983) Science 222:185-188.
- Perrone-Bizzozero NI, Finklestein SP, Benowitz LI (1986) Synthesis of a growth-associated protein by embryonic rat cerebrocortical neurons in vitro. J Neurosci 6:3721-3730.
- Neve RL, Perrone-Bizzozero NI, Finklestein S, Zwiers H, Bird E, Kurnit DM, Benowitz LI (1987) The neuronal growth-associated protein GAP-43 (B-50, F1): neuronal specificity, developmental regulation and regional distribution of the human and rat mRNAs. Brain Res 388:177-183.
- Neve RL, Finch EA, Bird ED, Benowitz LI (1988) Growth-associated protein GAP-43 is expressed selectively in associative regions of the adult human brain. Proc Natl Acad Sci USA 85:3638-3642.
- Benowitz LI, Apostolides PJ, Perrone-Bizzozero N, Finklestein SP, Zwiers H (1988) J Neurosci 8:339-352.
- Benowitz LI, Perrone-Bizzozero NI, Finklestein SP, Bird ED (1989) J Neurosci 9:990-995.
- Moya KL, Jhaveri S, Schneider GE, Benowitz LI (1989) Immunohistochemical localization of GAP-43 in the developing hamster retinofugal pathway. Journal of Comparative Neurology 288:51-58.
- Erzurumlu RS, Jhaveri S, Benowitz LI (1990) Journal of Comparative Neurology 292:443-456.
- Dani JW, Armstrong DM, Benowitz LI (1991) Neuroscience 40:277-287.
- Fitzgerald M, Reynolds ML, Benowitz LI (1991) Neuroscience 41:187-199.
- Reynolds ML, Fitzgerald M, Benowitz LI (1991) GAP-43 expression in developing cutaneous and muscle nerves in the rat hindlimb. Neuroscience 41:201-211.
- Schaechter JD, Benowitz LI (1993) Activation of protein kinase C by arachidonic acid selectively enhances the phosphorylation of GAP-43 in nerve terminal membranes. J Neurosci 13:4361-4371.
